Why gout isn’t just about beer and steak
Most people think gout is caused by eating too much red meat or drinking too much beer. But that’s only part of the story. The real problem is what happens inside your body when purines - the building blocks of DNA and RNA - get broken down into uric acid. When too much builds up, it forms sharp crystals in your joints, triggering sudden, crushing pain. This isn’t a one-time flare. It’s a chronic condition tied directly to how your body handles purines.
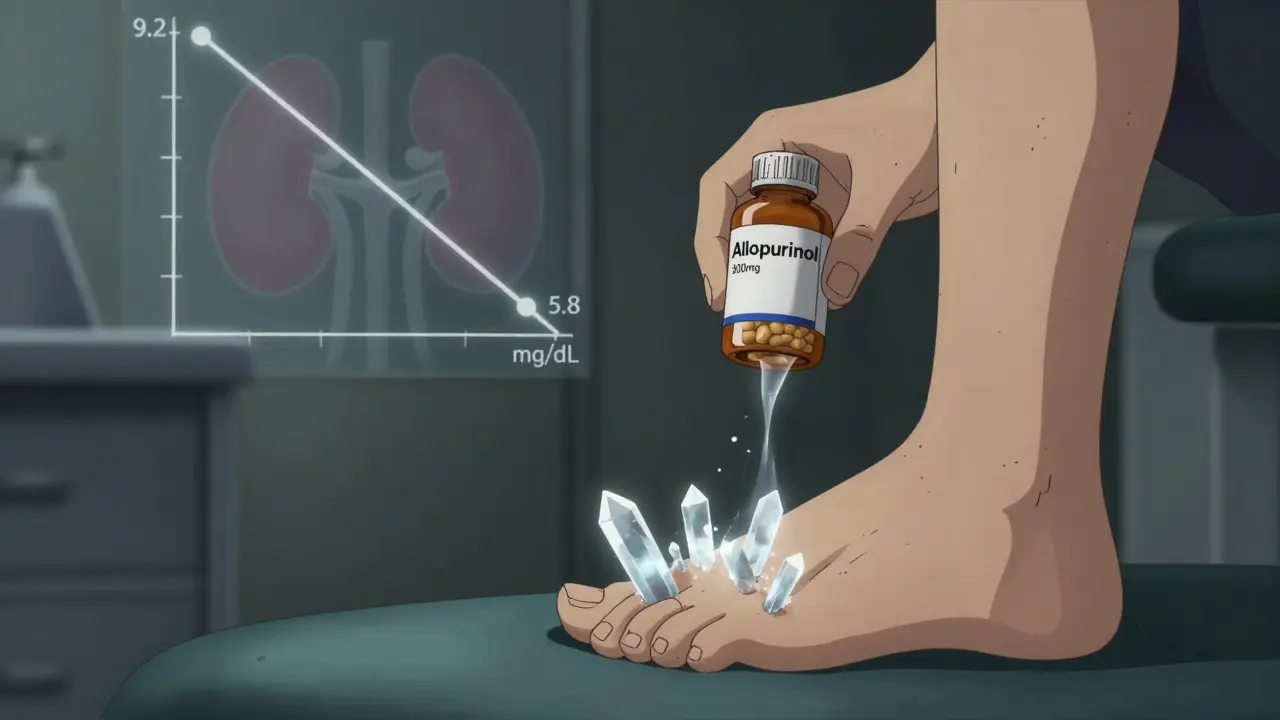
Every cell in your body contains nucleic acids. When they break down, they release purines. Your liver turns those into uric acid. Normally, your kidneys filter it out. But if you make too much, or your kidneys can’t clear it fast enough, uric acid levels rise. The magic number? 6.8 mg/dL. That’s the point where uric acid starts crystallizing in your joints. Once that happens, you’re not just at risk - you’ve already got gout.
How your body turns purines into pain
The path from purine to joint pain is precise. It starts with nucleotides breaking down into nucleosides. Guanosine becomes guanine, then xanthine. Adenosine becomes inosine, then hypoxanthine. In both cases, the final step is xanthine oxidase turning xanthine into uric acid. That enzyme is the key. Humans lost the ability to break down uric acid further millions of years ago. We’re stuck with it. No other mammal is.
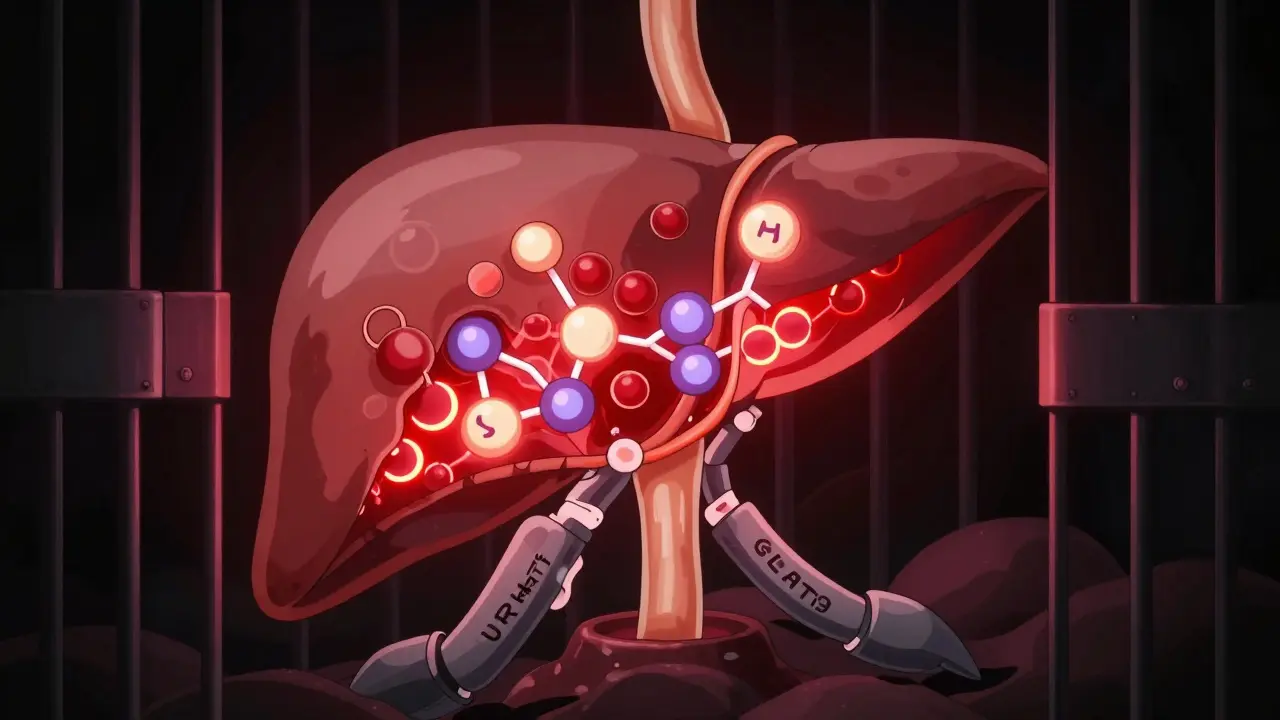
Here’s the twist: 65% of uric acid leaves through your kidneys. But 90% of what’s filtered gets reabsorbed. That’s thanks to two transporters - URAT1 and GLUT9. They’re like bouncers at a club, letting uric acid back in instead of out. Genetics play a big role here. Some people have variants in the SLC2A9 gene that make these transporters extra efficient, meaning their bodies hold onto more uric acid than others. That’s why two people eating the same diet can have wildly different uric acid levels.
And then there’s production. The enzyme PRPP amino transferase controls how much purine your body makes. If you have too much PRPP (a compound that fuels purine synthesis), or if your body doesn’t respond to feedback from IMP, GMP, or AMP, you end up overproducing. This happens in rare genetic disorders like Lesch-Nyhan syndrome - but even without that, some people just make more purines than others.
What urate-lowering drugs actually target
There are three main ways to lower uric acid: block its production, force it out through the kidneys, or break it down before it crystallizes. Each approach has different drugs, side effects, and costs.
- Xanthine oxidase inhibitors (XOIs) - These stop the final step: xanthine turning into uric acid. Allopurinol is the oldest and cheapest. It’s been around since 1966. Most patients start at 100 mg/day, then bump up by 100 mg every few weeks until uric acid hits below 6.0 mg/dL. But here’s the catch: 92% of patients only reach target when they take 300 mg or more. Most doctors never get there.
- Febuxostat - A newer XOI, approved in 2009. It works similarly but doesn’t need dose adjustments for kidney function. In trials, 66.7% of patients hit target at 80 mg/day. But the FDA slapped a black box warning on it in 2019 after the CARES trial showed more heart-related deaths than with allopurinol. It’s still used - but not for people with heart disease.
- Uricosurics - These drugs block URAT1 and GLUT9, so your kidneys dump more uric acid. Probenecid has been around since 1949. It works well if your kidneys are healthy, but it’s useless if your eGFR is below 50 mL/min. Lesinurad was approved in 2015 but pulled in 2019 because it damaged kidneys. New ones like verinurad are in Phase III trials and look promising.
- Uricase agents - Pegloticase is the only one approved in the U.S. It turns uric acid into allantoin, a harmless compound your body easily flushes. It’s powerful - 42% of patients hit target at 6 months. But it costs over $16,000 a month. And 26% have infusion reactions. You need premedication. It’s reserved for severe tophaceous gout when everything else fails.
Why most patients stop taking their meds
Even if you’re prescribed the right drug, you probably won’t stick with it. The Gout & Uric Acid Education Society found 61% of patients quit within a year. Why? Three reasons: they think it’s not working, they get side effects, or the dosing is too complicated.
Allopurinol users report rashes - 12% of them severe. Febuxostat users often see liver enzymes creep up. Both can trigger flares during the first few months. That’s not a sign the drug isn’t working - it’s a sign your body is dissolving crystals. But if your doctor doesn’t explain that, you’ll assume the medicine is making things worse. So you stop.
And then there’s the cost. Generic allopurinol costs $4.27 a month. Febuxostat? $59. Pegloticase? Over $16,000. Insurers fight it. Patients get denied. One Reddit user said they needed 17 prior authorization appeals just to get pegloticase. That’s not healthcare - that’s a bureaucratic marathon.
What your doctor should be doing - but often isn’t
Guidelines are clear. The American College of Rheumatology says: start low, go slow. Titrate allopurinol weekly. Monitor serum uric acid every 2-5 weeks until you hit target. Then check every 6 months. Use colchicine for the first 6 months to prevent flares.
But a 2024 study found only 29% of primary care doctors follow this. Most prescribe allopurinol at 100 mg, tell the patient to take it daily, and don’t check uric acid again for a year. That’s why so many people think the drug doesn’t work. They’re on too low a dose. Or they’re not monitored. Or they’re not told to expect flares.
And here’s another blind spot: diet. Yes, liver has 240-400 mg of purines per 100g. Anchovies have 500 mg. Beer has 10-20 g per liter. But dietary changes alone? They lower uric acid by maybe 1-2 mg/dL. That’s not enough. You can’t eat your way out of gout. Medication is non-negotiable.

The future: what’s coming next
There’s hope on the horizon. Verinurad - a new selective URAT1 blocker - showed 74% of patients hit target in early trials when paired with febuxostat. Results are expected in mid-2026. Arhalofenate, a dual-action drug that lowers uric acid and reduces inflammation, cut flare frequency by 58% in a 2024 trial.
Researchers are also looking at genetic markers. If you carry certain variants in SLC2A9, you might respond better to uricosurics. Others with HPRT mutations might need uricase from day one. Personalized treatment is coming.
But the biggest barrier isn’t science. It’s access. Gout is now the most common inflammatory arthritis in men over 65. In Asia-Pacific, it’s exploding. China alone has 23.7 million patients. Yet, in the U.S., only 37% of gout patients ever reach target uric acid levels. That’s not a treatment failure - it’s a system failure.
What you need to know right now
- If you have gout, your goal isn’t to avoid flares - it’s to get your uric acid below 6.0 mg/dL. For severe cases, aim for 5.0 mg/dL.
- Allopurinol is still first-line for most people. But you need to take enough. Most patients need 300 mg or higher.
- Don’t stop your medication because you have a flare. That’s normal. Use colchicine to protect yourself.
- Get your uric acid tested. Don’t wait a year. Check every 2-5 weeks until you’re at target.
- Diet helps, but it’s not enough. Medication is the only way to dissolve crystals and prevent joint damage.
Gout isn’t a lifestyle disease. It’s a metabolic disease. And it’s treatable - if you’re given the right tools, the right dose, and the right support. Too many people are left behind. You don’t have to be one of them.

Scott Conner
February 7, 2026 AT 09:57Tatiana Barbosa
February 8, 2026 AT 06:24Also, colchicine is your best friend during the first 6 months. Don't be scared of it. It's not poison. It's peace.
MANI V
February 9, 2026 AT 05:06I stopped all meds. Started drinking apple cider vinegar. Lost 40 lbs. No flares in 11 months. Your body heals itself. Science is just a tool to keep you dependent.
Susan Kwan
February 10, 2026 AT 15:37I'm calling my rheumatologist tomorrow to demand a uric acid test. I've been on allopurinol for 3 years and never had my levels checked. I'm not even mad. Just embarrassed.
Ryan Vargas
February 11, 2026 AT 08:37And yet, we prescribe dosages like we're tuning a radio. 'Turn it up until the static stops.' But what if the static is the signal? What if the inflammation is the immune system screaming, 'I'm still trying to fix this!'?
The system isn't broken. It was designed this way. To commodify suffering. To turn metabolic imbalance into a subscription service. We are not patients. We are revenue streams with joints.
Tasha Lake
February 11, 2026 AT 12:14Also, the SLC2A9 gene thing? Wild. My cousin has the variant that makes URAT1 super efficient. She eats salad and still has gout. Her diet? Perfect. Her genetics? Not so much. This post nailed it.
Simon Critchley
February 13, 2026 AT 07:55I had pegloticase last year. Cost me £12k in UK NHS time, but I got it. Had 3 infusions before they let me have it. Had to sign 7 forms, get 3 referrals, and a letter from my mum saying I'm 'not faking it'.
Now I'm uric acid at 4.8. No flares. No tophi. I can wear sandals again.
PS: Verinurad sounds like a superhero. I hope it's real.
Ritteka Goyal
February 13, 2026 AT 17:51My uncle died last year with a foot full of tophi. He never saw a rheumatologist. He just suffered. This post is true. But in places like mine? It's just words on a screen. We need pills. Not lectures.